

The Electronic energy is what you get from the FINAL SINGLE POINT ENERGY, then the Zero-Point Energy (ZPE) is calculated, together with the thermal correction, which has to do with the rotational, vibrational and translational degrees of freedom. It is the only liquid presented in this section. Water is one of the most common working fluids involved. In thermo-systems, many working fluids can be used. 0.00141627 Eh 0.89 kcal / mol Thermal translational correction. For most substances, thermodynamic properties are presented in the form of tables because they are too complex to be expressed by simple equations. 0.00247510 Eh 1.55 kcal / mol Thermal rotational correction. 0.10741046 Eh 67.40 kcal / mol Thermal vibrational correction. INNER ENERGY - The inner energy is : U = E ( el ) + E ( ZPE ) + E ( vib ) + E ( rot ) + E ( trans ) E ( el ) - is the total energy from the electronic structure calculation = E ( kin - el ) + E ( nuc - el ) + E ( el - el ) + E ( nuc - nuc ) E ( ZPE ) - the the zero temperature vibrational energy from the frequency calculation E ( vib ) - the the finite temperature correction to E ( ZPE ) due to population of excited vibrational states E ( rot ) - is the rotational thermal energy E ( trans ) - is the translational thermal energy Summary of contributions to the inner energy U : Electronic energy. For this, click on the drop-down menu associated with the Substance field. To make use of this tool: Select the substance that youre interested in studying. Multiscale NEB-TS for transition states Use the boiling point calculator to find the boiling point at a given pressure for different substances such as water, hydrogen, methane, or others.Starting with Multiscale models - QM/XTB ONIOM Calculate Properties Thermodynamic and Transport Properties of Water and Steam.Changing the temperature after the calculation.Thermodynamics at different temperatures.Hello water! Your first ORCA calculation.
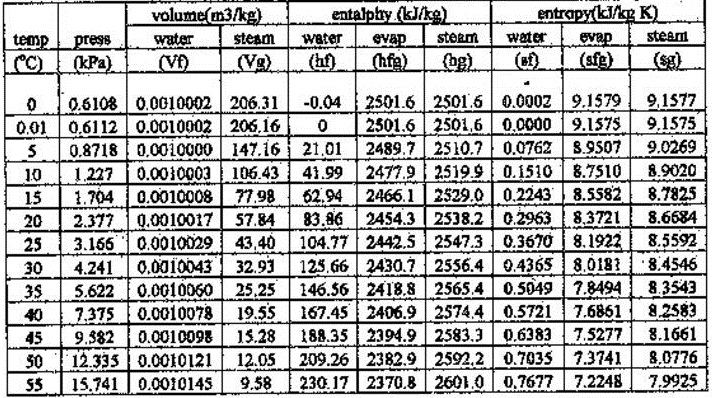
ThermodynamicData returns all properties with their values at STP. 1) select the name of the fluid you wish to calculate 2) select the calculation mode and enter the independent variables: always the pressure and either the temperature and the quality if the saturation temperature is set (P,T,x), or the enthalpy (P,h), or the entropy (P,s).Parameter values can also be supplied as an Association.As shown in Figure 12.6, Q is the net heat transferred into the system that is, Q is the sum of all heat transfers into and out of the system. 12.6 Here, U is the change in internal energy, U, of the system. Supported combinations of parameters include: In equation form, the first law of thermodynamics is U Q W.Parameters include "Density", "Enthalpy", "Entropy", "InternalEnergy", "Pressure", "Quality" and "Temperature".If a single parameter is specified, that value will be coupled with standard temperature (or pressure if the parameter is "Temperature") to derive a value. If not otherwise specified, physical properties are given for standard temperature and pressure (293.15 K and 101325 pascals).It is capable of calculating the properties density, specific isobaric heat capacity, specific isochoric heat capacity, specific enthalpy, specific entropy, specific internal energy, dynamic viscosity and kinematic viscosity as function of. Properties are returned using Quantity where appropriate. This calculator calculates thermodynamic properties for water as function of pressure and temperature.Properties that do not apply or are not known in a particular case are indicated by Missing.ThermodynamicData gives a list of all properties available.ThermodynamicData gives a list of the available substances.


 0 kommentar(er)
0 kommentar(er)
